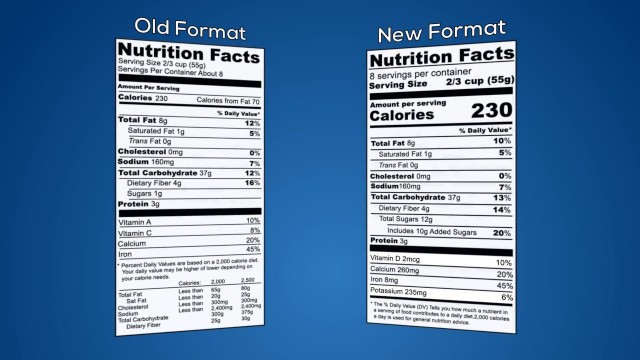

'Transcript: In 2016, the U.S. Food and Drug Administration finalized significant changes to food and beverage labeling. This video provides food labelers with an overview of a few of the most significant changes. FDA has updated the format of the Nutrition Facts chart. For example, the fonts for serving size and calories per serving are bigger and bolder, and the calories from fat have been removed. FDA also increased the font size for servings per container. Added sugars are now displayed on a separate line and represent a daily value. FDA added Vitamin D and Potassium to the required list of nutrients, while Vitamins A and C were made optional. The new rules also update the formulas for daily values of sodium, dietary fiber, and vitamin D. FDA now requires the amount of each nutrient to be declared in micrograms and milligrams in addition to the percent daily value. To better represent average U.S. consumption, FDA has changed what defines a serving size for certain foods. For example, many 20 ounce bottles of soda are currently labeled as 2 servings. The new rules require 20 ounce sodas to be labeled as one serving. Products that may be consumed in either one or multiple sittings may be required to bear a “dual column” label that identifies calorie and nutrient information for both “per serving” and “per package or unit”. Originally, the compliance deadline for most food labelers to incorporate FDA\'s new requirements on their labels was July 26, 2018. In September 2017, FDA proposed to extend the deadline to January 1, 2020 because industry expressed significant concern about their ability to update all their labels on time. The process requires a considerable amount of time, so companies should start the process of updating their food labeling as soon as possible. Most importantly, consider all of the new requirements thoroughly. Labeling mistakes can lead to costly relabeling and detentions. It\'s wise to have a professional review your redesigned labels before printing. Registrar Corp Regulatory Specialists can review your food label for compliance with FDA\'s new requirements. Clients receive a detailed report along with a revised print-ready label that incorporates our recommended changes. For more information about FDA food labeling requirements or Registrar Corp’s service, simply phone our US office at 757-224-0177 or go to our website at www.registrarcorp.com.'
Tags: Food , FDA , nutrition facts , Regulations , serving size , labeling , Food Labeling , food and drug administration , fda compliance
See also:


comments